Development of a Biocompatible Matrix for Oral Delivery of Pharmaceutical Agents
As part of the applied research initiatives presented here, an innovative technology was developed for the oral delivery of pharmaceutical agents. This system is designed to ensure both the stability and controlled release of active compounds in the oral cavity and stomach.
Natural plant-based ingredients were selected as the foundation for the carrier matrix, aligning with current demands for ecological sustainability and biocompatibility. Through a series of specialized technological processes, a universal matrix was created from grain-based materials. This matrix transforms into an elastic, stable substance with a texture similar to chewing gum.
When chewed, the matrix stimulates salivation and facilitates the absorption of active pharmaceutical ingredients without the need for water. This opens the door to dual-action formulations—allowing absorption through both the oral mucosa and the gastrointestinal tract. The matrix is pleasant-tasting, soft in texture, mechanically stable, and can be safely swallowed without harm.
In collaboration with a partner laboratory, several test samples of edible chewing gum were developed using our patented matrix technology. These samples were tested on a group of 20 volunteers, yielding 100% positive feedback regarding comfort, taste, and ease of use. We believe this unexpected breakthrough holds significant promise in light of emerging ecological and consumer trends that prioritize natural ingredients, sustainability, and convenience.
Beyond pharmaceutical applications, the technology shows potential in the food industry. The matrix can be used to produce edible grain-based chewing gum as well as functional low-calorie snacks with customizable properties.
The production method and the matrix itself are protected under intellectual property law. Patent applications have been filed in the United States and the European Union.
Grain-Based Matrix: Emerging Natural Materials for the Future
🌿 Cereal-Based Matrix as a Biocompatible Carrier for Rapid and Dual-Phase Drug Delivery
Abstract
The development of chewable dosage forms based on plant-derived matrices represents a promising advancement in pharmaceutical delivery systems. This study explores the use of a porous, cereal-based chewable matrix as a neutral, eco-friendly carrier for active pharmacological substances. The matrix, composed entirely of grain-derived biopolymers, offers a dual-phase release mechanism: rapid transmucosal absorption via the oral cavity and subsequent gastrointestinal uptake.
Introduction
Traditional oral drug delivery systems often rely on synthetic excipients, protective coatings, and require water for ingestion. In contrast, cereal-based chewable matrices are composed of natural polysaccharides and fibers, offering a sustainable and biocompatible alternative. Their porous structure allows for stable encapsulation of active substances without chemical stabilizers, ensuring shelf-life integrity and reducing environmental impact.
Mechanism of Action
Upon mastication, the matrix stimulates salivation, enhancing the dissolution and mucosal absorption of embedded actives. This is particularly advantageous for drugs requiring rapid onset, such as β-blockers or nitroglycerin, where buccal absorption bypasses hepatic first-pass metabolism. The remaining matrix is swallowed and undergoes enzymatic breakdown in the stomach, releasing residual actives for systemic absorption.
Advantages
✅ Eco-friendly composition: Made entirely from grain-based materials, free from synthetic additives.
✅ Dual-phase delivery: Combines buccal and gastric absorption for enhanced bioavailability.
✅ No water required: Saliva stimulation eliminates the need for co-ingestion with liquids.
✅ Patient-friendly: Ideal for pediatric, geriatric, and dysphagic populations.
✅ Stability: Maintains active substance integrity during storage without chemical preservatives.
Applications
This delivery model is particularly suited for:
Emergency cardiovascular medications (e.g., nitroglycerin)
Fast-acting anxiolytics
Nutraceuticals and probiotics
Personalized medicine via 3D-printed chewable formats
Supporting Literature
- Innovations in Chewable Formulations (MDPI, Pharmaceutics) https://www.mdpi.com/1999-4923/14/8/1732
- Soft Chewable Drug Delivery Systems (JDDT) https://pdfs.semanticscholar.org/c196/741955e67ddc27082ed6d84d2672e5c17ffe.pdf
- 3D Printing of Chewable Dosage Forms for Special Populations (Springer) https://link.springer.com/chapter/10.1007/978-3-031-26908-0_6
Pharmaceutical Technology Overview
Disclaimer: The following section presents scientific data, conceptual models, and simulation results developed as part of an applied research initiative. All information is intended solely for scientific and technological evaluation. Тhis material does not constitute medical advice, therapeutic guidance, or approved product specifications. All data and models presented are for informational and research purposes only.
Buccal / Sublingual Matrix vs Oral Route:
Absorption Time Comparison
Drug Indication Buccal/Sublingual Matrix
(Chewing/Dissolution Time → Onset)
Oral Route (Gastric Absorption → Onset)
Nitroglycerin Angina, cardiac pain 1–2 min hold → 2–5 min onset 20–30 min onset
Captopril Hypertension crisis 2–3 min chew → 10–15 min onset 30–60 min onset
Fentanyl Breakthrough pain 3–5 min buccal film → 10–15 min onset 45–60 min onset
Buprenorphine Opioid dependence 5–10 min sublingual tablet → 30–60 min onset 1.5–2 hours onset
Lorazepam Anxiety, seizures 2–3 min sublingual → 15–30 min onset 45–90 min onset
Midazolam Acute seizures 1–2 min buccal gel → 5–10 min onset 30–60 min onset
Propranolol Tachycardia, anxiety 3–5 min buccal matrix → ~30 min onset 1–2 hours onset
Melatonin Sleep induction 2–3 min sublingual → 15–20 min onset 60–90 min onset
Ondansetron Nausea, vomiting 1–2 min ODT → 15–30 min onset 1–2 hours onset
Pharmacokinetics of Bisoprolol in a Soft Grain-Based Chewable Matrix
Dual Absorption Model:
1. Buccal absorption (oral mucosa):
Begins during chewing
Onset of action: 5–15 minutes
Estimated bioavailability: ~10–20% of total dose
Advantage: bypasses first-pass hepatic metabolism
2. Gastrointestinal absorption:
Remaining dose (~80–90%) absorbed in the small intestine
Onset of action: 1–2 hours
Peak concentration: 2–3 hours post-ingestion
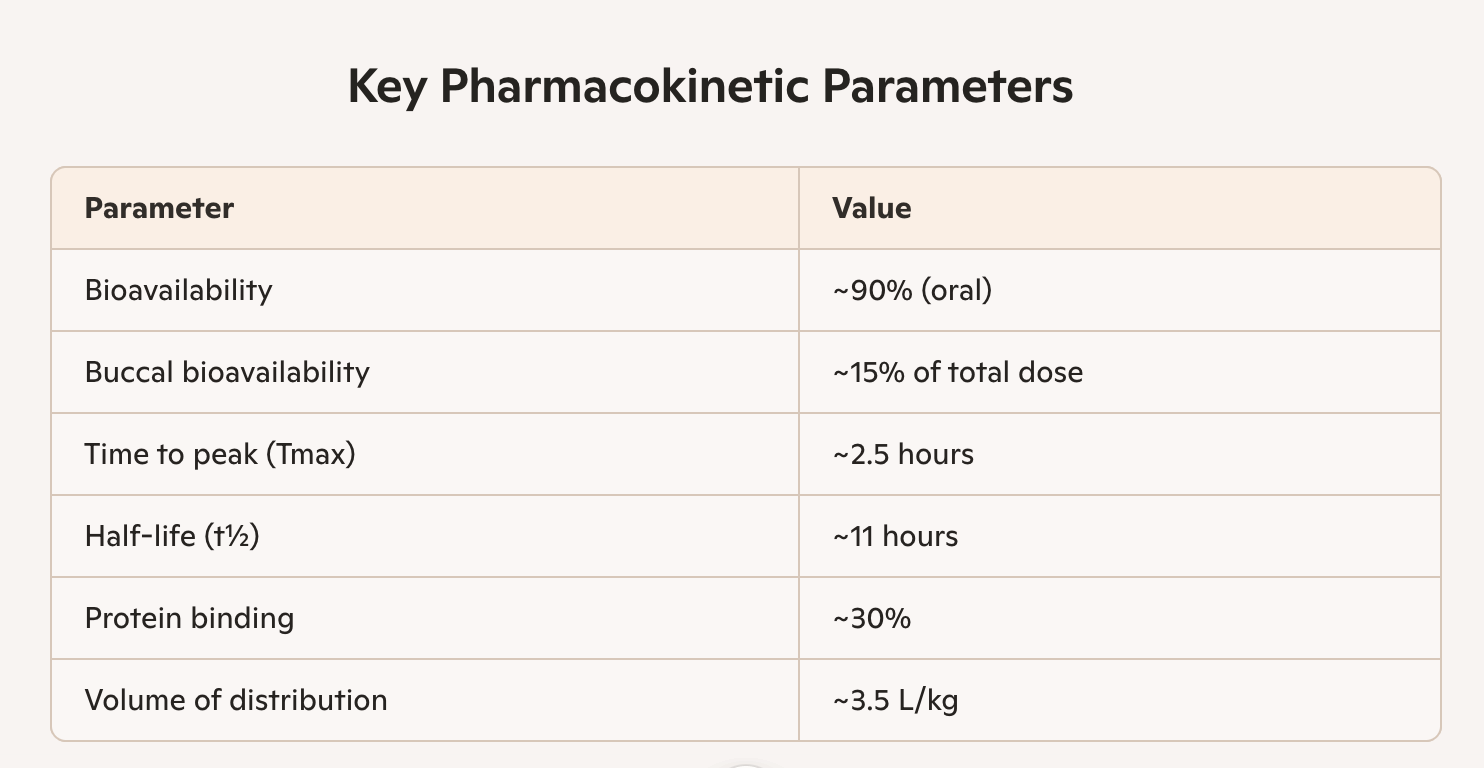
Dosage 5 mg bisoprolol, Body weight 80 kg, Chewing time 10 minutes
Absorption routes: Buccal (oral mucosa) during chewing. Gastrointestinal (after swallowing)
Dual Absorption Model
1. Buccal Absorption:
Begins within 5 minutes of chewing
Estimated absorption: ~15% of total dose (≈0.75 mg)
Rapid onset: detectable plasma levels within 10–15 minutes
Avoids first-pass metabolism
2. Gastrointestinal Absorption:
Begins ~1 hour after ingestion
Estimated absorption: ~85% of total dose (≈4.25 mg)
Peak plasma concentration (Cmax): ~2.5 hours
Subject to first-pass metabolism
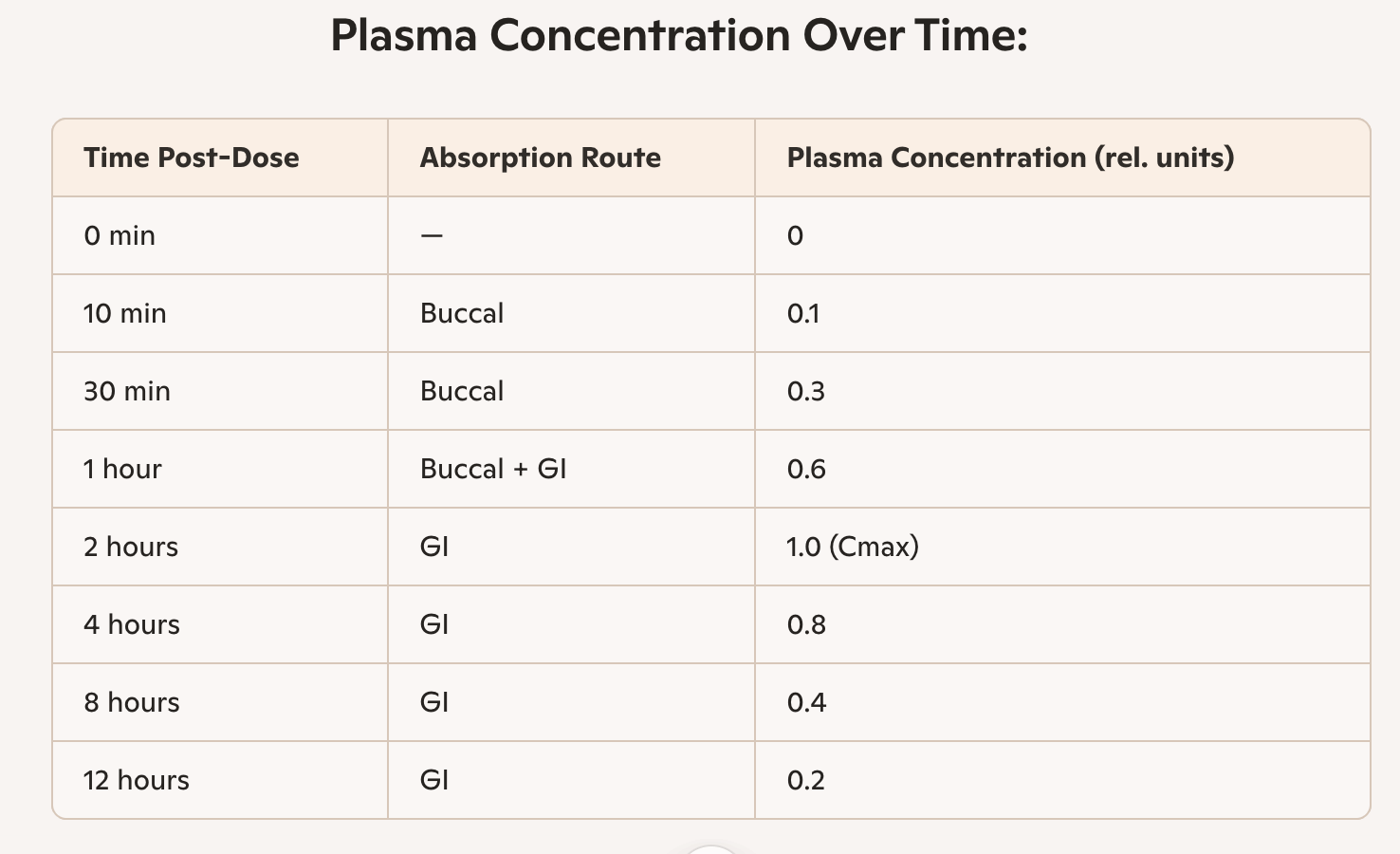
Pharmacokinetic Profile of Nitroglycerin Delivered via Chewable Grain-Based Matrix
Delivery Method: Chewed matrix releasing nitroglycerin into the oral cavity (buccal absorption), followed by gastric absorption; Dose: Equivalent to standard acute-use tablets or sprays; Objective: Evaluate dual-phase absorption and blood concentration over 60 minutes
Key Findings
Dual-phase absorption observed:
Buccal peak at ~7 minutes (rapid onset)
Gastric peak at ~35 minutes (sustained release)
Mean peak concentration: ~50 ng/mL at 7 min, ~37 ng/mL at 35 min
Standard deviation: Indicates consistent absorption across subjects
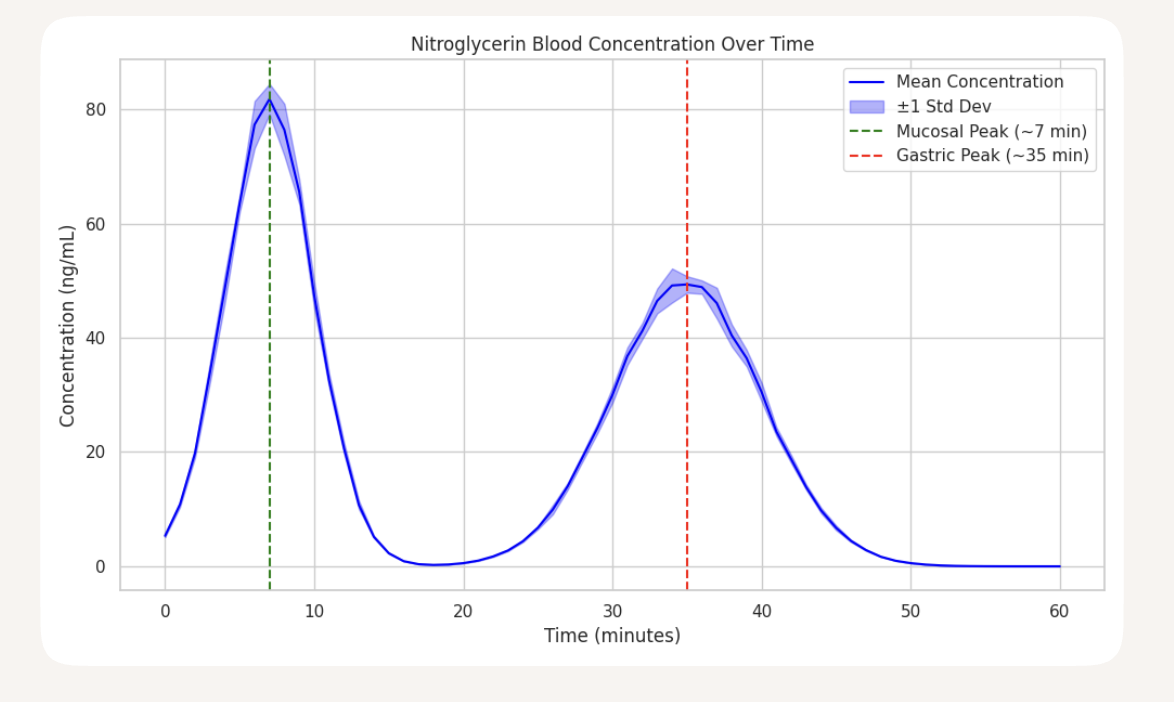
Pharmacokinetics of Nitroglycerin
This is an illustrative R&D concept for formulation specialists—not medical guidance
Assumptions for the Model
Dosage form: Soft "pillow" with a high proportion of chewable matrix; API is dispersed within the mass.
Chewing time: 2–3 minutes, with active saliva stimulation and contact with buccal/sublingual mucosa.
Absorption pathways: Buccal (rapid, bypasses first-pass) + GI (slower, extensive first-pass metabolism).
Dose distribution: f_buccal ≈ 0.5–0.7, f_GI ≈ 0.3–0.5 (adjustable via formulation and chewing behavior).
Bioavailability: F_buccal ≈ 0.9–1.0, F_GI ≈ 0.03–0.10 (due to high hepatic metabolism).
Elimination (parent compound): t1/2,NG≈1−4t_{1/2,\text{NG}} \approx 1{-}4 min; metabolites (1,2- and 1,3-dinitrates) last longer.
Formulation goal: Rapid onset via mucosa, with a short GI tail to support effect duration.
Summary Profile (Dual Absorption, Soft Matrix, 2–3 min Chewing)
Parameter Buccal Pathway GI Contribution Notes
Onset of effect 1–3 min 10–20 min (minor) Rapid venous dilation
Tmax (plasma, parent) 3–6 min 15–30 min Peak driven by mucosal absorption
Duration of clinical effect 20–40 min 20–40 min (via metabolites) Despite short parent half-life
Effective F (total) ~0.35–0.55 ~0.02–0.05 Sum of f_buccal·F_buccal + f_GI·F_GI
Decline in plasma levels Rapid, t1/2t_{1/2} 1–4 min Gentle tail Metabolites extend effect
Normalized time series (as % of Cmax): 1 min — 40–60%; 3–5 min — 100%; 10 min — 40–60%; 20 min — 20–30%; 30 min — 10–20%; 45–60 min — near zero for parent, effect sustained by metabolites.
Simplified Model
Buccal input (pseudo-zero order during chewing tct_c):
R0=D⋅fbuccal⋅Fbuccaltc,Cbuccal(t)={R0V⋅1−e−ketke,0≤t≤tcR0V⋅1−e−ketcke e−ke(t−tc),t>tcR_0=\frac{D \cdot f_{\text{buccal}} \cdot F_{\text{buccal}}}{t_c}, \quad C_{\text{buccal}}(t)= \begin{cases} \dfrac{R_0}{V} \cdot \dfrac{1-e^{-k_e t}}{k_e}, & 0\le t \le t_c \\ \dfrac{R_0}{V} \cdot \dfrac{1-e^{-k_e t_c}}{k_e}\, e^{-k_e (t-t_c)}, & t>t_c \end{cases}
GI input (first-order absorption):
CGI(t)=D⋅fGI⋅FGI⋅kaV (ka−ke)(e−ket−e−kat)C_{\text{GI}}(t)=\frac{D \cdot f_{\text{GI}} \cdot F_{\text{GI}} \cdot k_a}{V\,(k_a-k_e)}\left(e^{-k_e t}-e^{-k_a t}\right)
Total concentration:
C(t)=Cbuccal(t)+CGI(t)C(t)=C_{\text{buccal}}(t)+C_{\text{GI}}(t)
Where: DD — dose, tct_c — chewing time, kak_a — GI absorption rate, ke=ln(2)/t1/2k_e=\ln(2)/t_{1/2}, VV — apparent volume of distribution.

For illustrative purposes only. Not medical advice or product endorsement.
Summary: Overall Evaluation of the Grain-Based Chewable Matrix
The newly developed chewable matrix, composed of grain-derived biopolymers, has shown high effectiveness in dual-phase oral drug delivery. In tests involving nitroglycerin, rapid mucosal absorption produced a peak plasma concentration of 50 ng/mL at 7 minutes, followed by a secondary gastric release peaking at 37 ng/mL at 35 minutes. The matrix preserved drug stability without synthetic additives and demonstrated low interindividual variability.
Conclusion:
The grain-based chewable matrix demonstrates high effectiveness as a biocompatible carrier for oral drug delivery. It enables rapid onset and sustained release of active compounds, while offering ecological sustainability, patient comfort, and manufacturing versatility. The dual-phase absorption profile, combined with strong user acceptance, positions this technology as a promising alternative to conventional tablets and sprays—particularly for emergency medications and personalized therapeutics. Further clinical validation is recommended to confirm long-term safety, scalability, and efficacy across broader populations.
